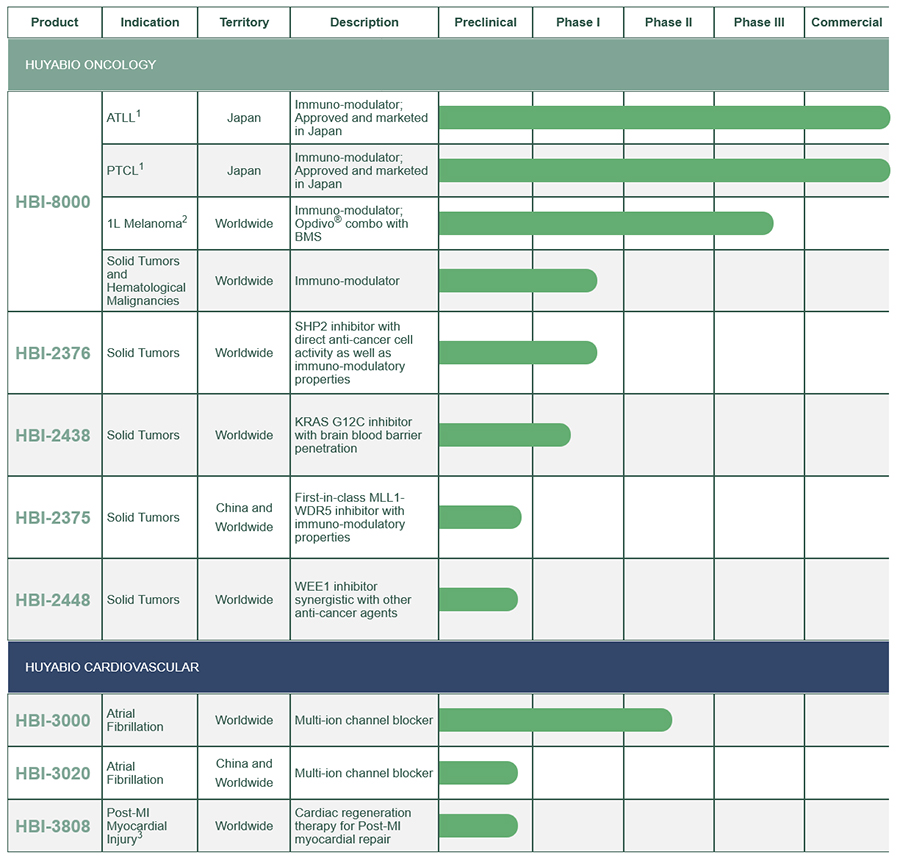
HUYABIO Development Compounds
To replenish the pipeline, HUYABIO identifies and selects a subset of promising early-stage compounds from its database engine and positions them for development in different markets worldwide. HUYABIO seeks opportunities in therapeutic areas with significant market potential and unmet medical needs, where the compound has a competitive advantage with strong patent position worldwide, and a clear clinical development path.
The company is currently developing a number of programs in two therapeutic areas including: 1) oncology programs; HBI-8000 HDAC inhibitor with immune modulatory properties, HBI-2376 the novel SHP2 inhibitor for solid tumors, HBI-2438 a differentiated KRAS G12C inhibitor, HBI-2375 a first-in-class MLL1-WDR5 inhibitor for leukemia or solid tumors; and 2) cardiovascular programs; HBI-3000/3020 a novel anti-arrhythmic compound, and HBI-3808, a small molecule with regenerative properties for myocardial infarction.
1 Partnered with Meiji Seika Pharma | ATLL – Adult T-cell Leukemia/Lymphoma | PTCL – Peripheral T Cell Lymphoma
2 Clinical collaboration with BMS; BMS is responsible for supply of Opdivo® for the trial, which is sponsored by HUYABIO
3 Post-MI – Post Myocardial Infarction
* Worldwide: ex-China global territory
About HBI-8000
- A selective Class 1 histone deacetylases inhibitor, orally administered, approved for treatment of T-cell lymphoma as monotherapy in China (2014) and Japan (2021), and in breast cancer as combination therapy with aromatase inhibitor in China (2019) and Taiwan (2023).
- Pharmacologically, it boosts immune response against cancer cells by epigenetic modification and converts tumor microenvironment from cold (inactive) to hot (active).
- Preclinical research has demonstrated its immune modulatory effects is of particular importance. It has the potential to amplify the benefits of therapies in immune oncology including, but not limited to immune checkpoint inhibitors, where functionality of immune cells and cancer antigen presentation are drivers for treatment success.
About HBI-2376
- SHP2 is an attractive target in oncology drug development due to its dual mode of actions to directly promote tumor growth via induction of signaling pathways in cancer cells and acts as a checkpoint molecule to suppress immune system in the tumor microenvironment. In preclinical studies HBI-2376 demonstrated its immune-modulatory property as well as direct anti-cancer activity in EGFR or KRAS mutant cancer cell lines and was superior compared to Novartis TNO-155 (Novartis Pharmaceuticals) and RMC-4550 (Revolution Medicines).
- HBI-2376 has broad indications in EGFR/KRAS mutant tumors such as NSCLC (non-small cell lung cancer), CRC (colorectal cancer) and HNSCC (head and neck squamous cell carcinoma) and in combination with EGFR/KRAS inhibitors. Additionally, HBI-2376 has a great potential for combination with checkpoint inhibitors, anti-PD1, anti-PD-L1 and anti-CTLA4 antibodies, in IO (immuno-oncology) indications.
- HUYABIO advanced HBI-2376 in Phase 1 studies in the clinic and is planning to test HBI-2376 in combination with other modalities in Phase 2 studies.
About HBI-2438
- KRAS G12C mutation is a well know driver of tumorigenesis in several indications such as NSCLC, CRC and pancreatic cancer and has been one of the challenging targets in oncology drug development. Recent approval of sotorasib (Amgen Inc) opened new treatment for patients with KRAS G12C mutation.
- Preclinical studies demonstrated good efficacy and safety for HBI-2438 as monotherapy or in combination with other anti-cancer agents. HBI-2438 is a well differentiated KRAS G12C inhibitor due to its ability to cross the blood brain barrier. In a preclinical model of brain metastasis, HBI-2438 significantly regressed growth of metastatic lesions carrying KRAS G12C mutation.
- HUYABIO successfully completed HBI-2438 IND submission and is currently testing it in Phase 1 clinical studies.
About HBI-2375
- HBI-2375 is the first-in-class MLL1-WDR5 epigenetic inhibitor capitalizing on HUYABIO expertise with epigenetic drugs including HBI-8000. MLL1-WDR5 interaction is critical for MLL1 histone methyl transferase activity to regulate expression of downstream genes involved in the progression of leukemias or solid tumors.
- HBI-2375 inhibited MLL1-WDR5 activity in vitro and in preclinical models. During early characterization HUYABIO discovered MLL1-WDR5 interaction is also involved in regulating activity of immune cells in tumor microenvironment as combination of HBI-2375 and anti-PD1 antibody significantly inhibited growth of solid tumors.
- Another unique feature of HBI-2375 is its ability to cross the blood brain barrier potentially targeting primary/metastatic lesions in the brain.
- HUYABIO has worldwide rights on HBI-2375 for development across the globe and has completed all the preclinical and CMC studies making it completely ready for IND submission.
About HBI-2448
- WEE1 plays important roles in regulating cell cycle in eukaryotic cells and serves as a key mediator of DDR (DNA damage response) in non-malignant cells to inhibit cell cycle progression and proliferation when DNA content is altered. WEE1 is highly expressed in a variety of indications such as lung, head & neck, urothelial, testis, cervical and lymphoma cancers. WEE1 Inhibition results in the progression of cell cycle and increased sensitivity to DNA damage agents such as radiation. Therefore, WEE1 inhibition in combination with other DNA damage agents provides an alternative treatment for cancer patients.
- HBI-2448 is a novel WEE1 inhibitor with good safety and efficacy in preclinical models as single agent or in combination therapy. HUYABIO is currently completing HBI-2438 required non-clinical and CMC studies for IND submission to regulatory agency.
About HBI-3000 and HBI-3020
- HBI-3000 and HBI-3020 are multi-ion channel blockers in development for the treatment of cardiac arrhythmias. Their initial indication is atrial fibrillation (AF), a condition that affects millions globally with incidence rates expected to rise as populations age. The CDC estimates that 12.1 million people in the U.S. alone will have AF by 2030. While many current AADs are effective in controlling arrhythmias, they also may pose serious risks including fatal arrhythmias and sudden death.
- The active form of HBI-3020 and HBI-3000 is a new antiarrhythmic drug (AAD) that inhibits multiple cardiac ion channels (INa-peak, INa-late, ICa,L, and IKr (hERG)) with uniquely similar potencies. Its preclinical pharmacology supports strong potential for treatment of both acute and chronic AF with distinct mechanistic and clinical advantages over current AADs.
- HBI-3000 is an intravenous form of the drug that was well-tolerated in two Phase 1 trials of volunteer subjects and Stage A of a Phase 2 in acute AF patients, and HBI-3020 is the second-generation oral form being developed for maintenance therapy of chronic AF.
About HBI-3808
- HBI-3808 is a novel orally bioavailable small molecule with the potential to provide an acellular alternative to stem cell administration and differentiation therapies in post-myocardial infarction (MI) cardiac tissue repair and regeneration.
- Pharmacology studies in rat LAD ligation model, a permanent coronary vessel ligation study (not ischemia/reperfusion), confirmed beneficial effects of HBI-3808, myocardium and regional and global cardiac function after MI.
- Mechanistic studies indicate that HBI-3808 acts directly on endogenous cells to promote cardiomyocyte formation and also supports cardiac tissue repair via induction of angiogenesis.
Back to Top